You are here
Pfizer to seek authorization for 2nd Covid booster for people 65 and older
Primary tabs
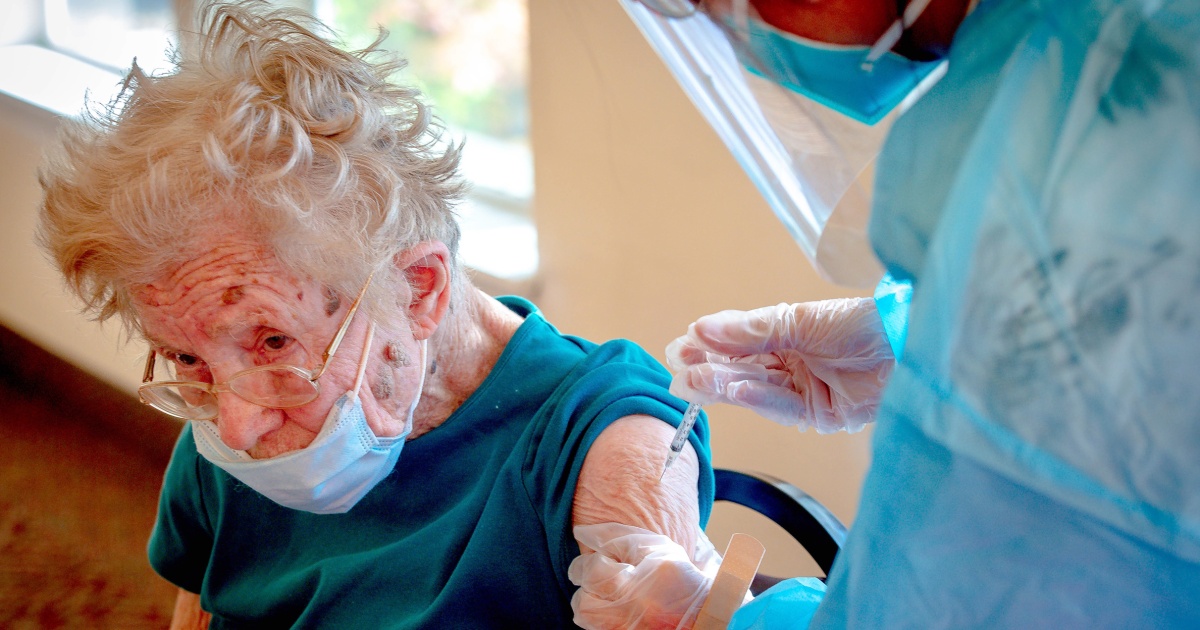 Pfizer asks FDA to authorize 2nd Covid booster for people 65 and older If the FDA authorizes it, the additional shot would go to people who are among those with the highest risk of serious illness. NBC News
Pfizer asks FDA to authorize 2nd Covid booster for people 65 and older If the FDA authorizes it, the additional shot would go to people who are among those with the highest risk of serious illness. NBC News Pfizer and BioNTech are expected to seek U.S. authorization this week for a second Covid-19 vaccine booster for people 65 and older, according to a person familiar with the plans.
If the Food and Drug Administration grants authorization, the additional shot would go to a group of people who are among those with the highest risk of serious illness and death from Covid.
The FDA has authorized booster shots for everyone 12 and older on an emergency use basis.
Multiple studies have shown that the protection from the initial booster dose begins to wane after several months, particularly against the omicron variant of the coronavirus.
Pfizer CEO Albert Bourla said Friday that the company was close to submitting data to the FDA on a fourth dose of its vaccine after its scientists found that the protection from the first booster began to wane after three or four months. ...



Recent Comments