You are here
Tue, 2022-08-23 18:22 — mike kraft
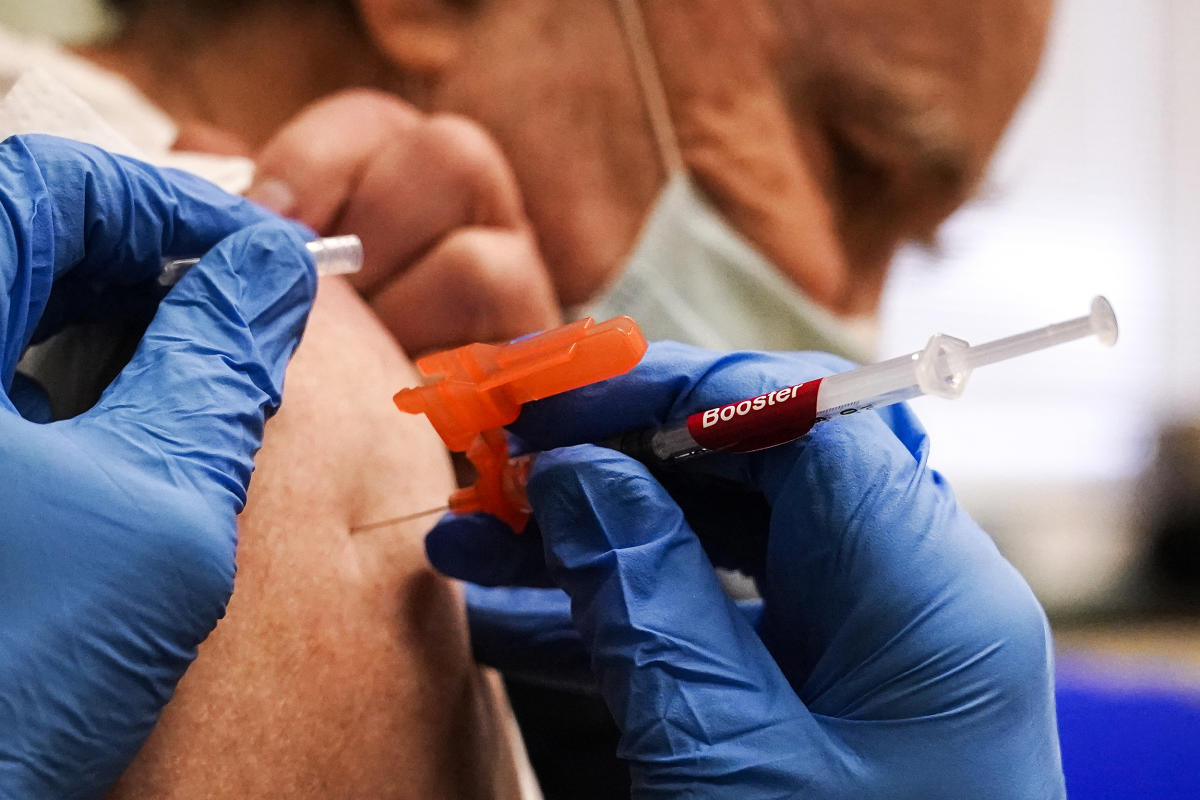 Moderna asks FDA to authorize its updated Covid booster shot Moderna asked the Food and Drug Administration to authorize its bivalent omicron Covid booster. YahooNews
Moderna asks FDA to authorize its updated Covid booster shot Moderna asked the Food and Drug Administration to authorize its bivalent omicron Covid booster. YahooNews
 Moderna asks FDA to authorize its updated Covid booster shot Moderna asked the Food and Drug Administration to authorize its bivalent omicron Covid booster. YahooNews
Moderna asks FDA to authorize its updated Covid booster shot Moderna asked the Food and Drug Administration to authorize its bivalent omicron Covid booster. YahooNews ...
The drugmaker’s application to the FDA, which covers adults ages 18 and older, follows a similar request from Pfizer-BioNTech on Monday. Pfizer is seeking authorization for everyone 12 and older.
Like Pfizer’s updated booster, Moderna’s so-called bivalent vaccine is specifically designed to target omicron subvariants, BA.4 and BA.5, as well as the original coronavirus strain in a single shot. The move follows a recommendation from the FDA in June to modify the vaccines to target the two subvariants.
...
Country / Region Tags:
General Topic Tags:
Problem, Solution, SitRep, or ?:
Groups this Group Post belongs to:
- Private group -



Recent Comments